- December 15, 2025
-
-
Loading

Loading
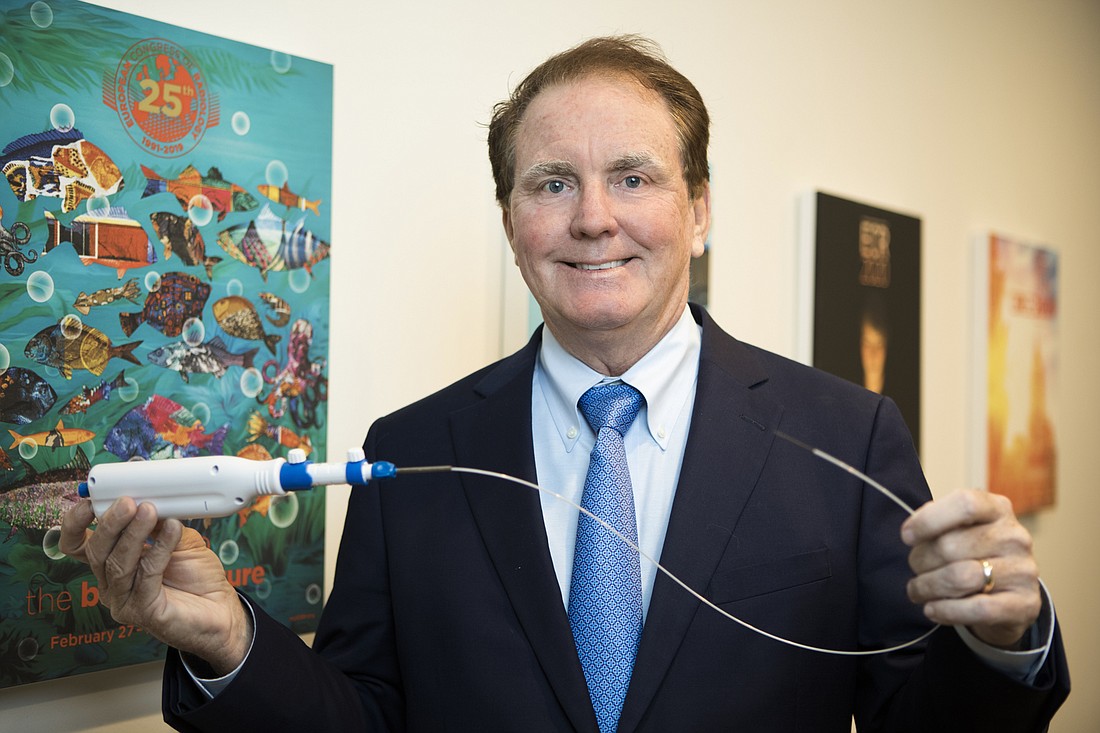
Dr. John Fisher, an interventional radiologist at Morton Plant Hospital in Largo and Mease Countryside Hospital in Safety Harbor, has created CytoCore, a medical device that makes biopsies faster, more efficient and less invasive for patients.